The Personal Care Industry, Challenges in Cleaning, Part II
In a market driven by innovation, where every year a quarter of all cosmetic products are new or improved versions, personal care manufacturers strive to define and implement suitable cleaning and disinfection procedures, especially when dealing with a high number of different formulations in one site.
The optimization of the cleaning process is critical for decreasing operational costs, downtime and for achieving the sustainability goals set by the company (such as the reduction of water, chemistry, and energy consumption).
The challenges commonly faced by the industry were described in the first article of this two-part series. The aim of this second article is to provide an initial guidance on how to optimise cleaning procedures, from the choice of chemistry, to the correct setup of the equipment available on site, and all while compliant with the requirements set by the industry.
1. Initial guidance for an optimised cleaning procedure
Any hygiene procedure on a personal care site requires cleaning process understanding to ensure reliable and consistent cleaning performance. The objective of section 2.1 is to provide an initial guidance on the parameters that have an impact on the cleaning results, that should be tracked during the cleaning operation.
In section 2.2, a more in-depth overview of the different cleaning methods is provided, as well as some indications on how to best optimise the cleaning procedures, taking into consideration the possible limits of the equipment available onsite.
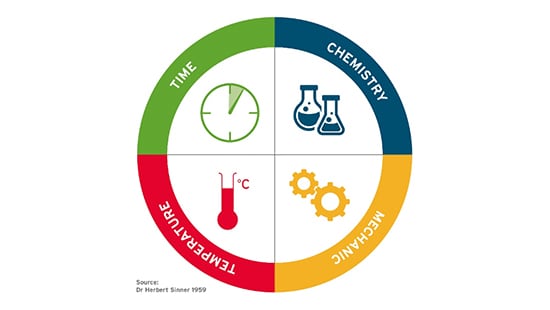
2.1 Basics on cleaning parameters — Sinner Circle
Parameters influencing cleaning are simply described within the Sinner Circle1.
To achieve a right first time cleaning, the effects of the four parameters must form the complete circle, compensating for each other; when one segment is less effective, the others compensate. Below some considerations on each of these parameters:
- Temperature
- In general, the quality of cleaning results increases with a higher temperature. Trends for saving energy and worker safety led to efforts to lower the temperature for cleaning.
- Lower cleaning temperatures are also recommended for some product residues such as, starch and other carbohydrates.
- This can be circumvented by using an automated cleaning system allowing for free selection of the cleaning temperature.
- New state-of-the-art formulations of personal care cleaning agents even allow for cleaning of cosmetic products (also with high pigment concentrations), within a lower temperature range (60-70 degrees Celsius).
- Time
- The need for increased production capacities is pushing the industry to optimise cleaning cycles. Cycles that were developed in the past tend to have long cleaning times to ensure quality. Those cleaning cycles were never optimised to increase productivity.
- Multiple, short cleaning cycles are to be preferred for products containing TiO2/FeOx to avoid shadowing in the equipment.
- Different cleaning times can be expected for cleaning during campaigning (cleaning between batches of the same product) or cleaning between different products2
- Chemistry
- Specifically developed chemistry is recommended to remove residues from personal care products. The typical soiling requires high levels of detergency and other cleaning active ingredients (e.g. solubilizer, complexing agents and many more).
- It is not recommended to use standard products form Food and Beverage industries as these cleaning products are designed to clean residues that contain much less hard to clean residues, as mentioned in the section entitled “Specific cleaning challenges in Personal Care industry” in the first article of this series.
- Mechanical Action
- Mechanical action is often defined by the equipment instrumentation. A wide variety of operations can be found in the industry, from dedicated CIP systems designed for a defined product, to simple vessels without any cleaning equipment which are cleaned by soaking them in their entirety.
- Even with automated processes, it is seen in practice that a lot of manual cleaning applications are still used. This manual cleaning varies from high pressure lances used for washing, to the use of a water hose for rinsing or using tools like sponges, brushes and mops inside the manufacturing equipment. All these different applications have different mechanical actions and operator safety should be considered when determining the manual cleaning process needed.
2.2 The main cleaning methods: indications for an optimised procedure
- CIP Cleaning
CIP (Clean in Place) in a recirculation system is an industry standard today. The CIP fluid is recirculated across the object ensuring constant temperature, flow and chemical concentration. CIP cleaning of equipment or pipeline circuits is defined as cleaning without disassembling or opening of the equipment, and with little or no involvement on the part of the operator. One CIP system can deliver cleaning solutions to different objects.
CIP cleaning is all about energy transfer. Within a CIP programme, the right energy to remove the soiling from the equipment needs to be identified.
Cleaning that uses the optimal time, temperature, flow rates and amount of detergent is the result of an engineered CIP cleaning cycle. The basis parameters of an effective cleaning regime can be analyzed with laboratory bench scale studies. These studies identify which detergent delivers the most effective cleaning, at what temperature and for how long. The parameters gained from laboratory trials are usually the basis for developing the full CIP cycle.
Validation of an effective CIP cleaning is easily achievable, because the cleaning process is identical every time. To keep this constant a change control as well as maintenance for the CIP system itself should be installed.
Reusing rinse water within the cleaning process is also an option, allowing for a more ecological and economical process.
Adding a CIP system to existing equipment can necessitate a high investment and can be difficult to execute, which can be considered a disadvantage of such a system.
- Cleaning by Soaking
When CIP cleaning is not possible, the solution for harder to clean residues is to soak the equipment with a detergent solution and use the agitator of the vessel to introduce mechanical action to the cleaning cycle. The disadvantage of this method is the increased consumption of chemistry, time and water. Therefore, recirculating the water and the cleaning solution within the object being cleaned, to keep the consumption of water, energy and chemicals down to a minimum level, is recommended.
- COP Cleaning
COP (Clean out of Place) cleaning is encountered for easy to clean products. Operators use medium or high-pressure washers to clean out the production equipment by hand. The disadvantages here are the fact that the cleaning performance depends on the operator, lack of temperature and pressure on larger equipment and limited detergent possibilities to ensure worker safety.
The use of a parts washer to clean utensils and critical parts of the equipment in a COP application is preferred over manual cleaning. The cleaning programme of a parts washer is independent of the operator and will be flexible in time, temperature, detergent concentration and water usage.
- Manual Cleaning
Manual cleaning is very easy to set up but comes with many challenges. The temperature (max 45°C) and choice of detergent (neutral pH-range) limit the performance. The cleaning is time consuming and labour intensive. Consistent cleaning is difficult to achieve due to the operator variation and difficulty of monitoring.
- Summary of Cleaning Methods
A table summarizing the key advantages and disadvantages of the different hygiene processes is shown below:
|
Parameter |
Manual cleaning |
COP / Soaking application |
CIP process |
|
Temperature |
Ambient – 45°C Hot water from tap might be too high in temperature |
Ambient – 95°C Consider time needed to fill the object and heat up to required temperature |
Ambient – 95°C No time loss as solution is stored at use temperature |
|
Chemicals used |
Manual detergents Consider operator safety |
Manual and CIP detergents Detergents dosed into system manually (consider operator safety) |
CIP detergents Automatic dosing therefore safe process |
|
Concentration |
Manually dosed (some operators consider more detergent = better cleaning) |
Manually dosed (some operators consider more detergent = better cleaning) |
Automatic dosing Concentration can be tracked by e.g. conductivity (to facilitate validation) |
|
Time |
Often quick cleaning process but complete dismantling required |
Long cleaning time due to fill up the equipment completely and get to cleaning temperature |
Quick cleaning as only the time needed for cleaning is used (no heat up etc) |
|
Mechanical action |
High By using sponges, brushes, mops etc but operator dependent |
Low Often only agitator can be used and additional manual cleaning needed |
Medium – High If correct CIP tools used (can even be controlled by pressure check at spray device) |
|
Validation |
Hard to achieve Manual cleaning processes should be verified at frequent intervals |
Hard to achieve A lot of manual documentation required to capture cleaning times, concentrations and temperatures |
Achievable Main cleaning parameters are documented by the CIP system automatically |
Table 1: Overview of different cleaning applications and process parameters
Summary
The combination of chemistry, temperature, cleaning time and mechanical action directly impacts the results of the cleaning. Some of these parameters are limited by the equipment available onsite and they should all be tracked during the cleaning operation, to identify possible inefficiencies that can lead to high operational costs, long downtime, high water and energy consumption. These inefficiencies can be addressed to hygiene partners like detergent suppliers that can support the manufacturer with the optimization of the cleaning procedures, from the choice of the chemistry to the implementation onsite, according to the cleaning applications available.
Peer Review
The authors wish to thank our peer reviewer Paola Piantanida for reviewing this article and for providing insightful comments and helpful suggestions.
References
1. Zeitschrift Getränkeindustrie 11/2004: Der Sinner'sche Kreis: Basis einer erfolgreichen Reinigung und Desinfektion.
2. American Society for Testing and Materials E3106 "Standard Guide for Science-Based and Risk-Based Cleaning Process Development and Validation" www.astm.org.
_



