Spotless Standards: Mastering Contamination Control in Personal Care and Cosmetics
Based on an article originally published in the June 2025 issue of Personal Care Magazine.

Establish a repeatable and clear procedure to ensure you accurately pass your visually clean assessment across different operators.
Imagine that a single lapse in your cleaning procedure tarnished your brand’s reputation in just a matter of days. From what seemed to be a simple misstep in your cleaning and sanitization (C&S) procedure, you are now faced with a product recall and press release to warn consumers, a formal root cause analysis, and a corrective action plan, among many other responsibilities. The financial and reputation costs are staggering, with potential FDA findings adding to the burden. You have now dedicated hours, days, or even weeks to rectify an issue that could have been easily prevented with proper C&S controls. In the world of cosmetics, ensuring spotless equipment isn’t just about compliance—it’s about safeguarding consumer trust and health.
For the most part, regulations worldwide require that Personal Care and Cosmetics products not be adulterated or contaminated. The manufacturer must meet these regulations by controlling critical steps in the manufacturing process to ensure that contamination does not occur. Various controls can be put in place in the manufacturing and packaging processes, cleaning processes, employee hygiene, plant hygiene, raw material controls and employee training, to name a few. The release of the draft Current Good Manufacturing Practices (cGMPs) of the Modernization of Cosmetics Regulation Act (MoCRA) is still pending, and with that comes speculation about what will or will not be required. The international standard of GMPs for cosmetics, ISO 22716:2007, can be referenced as an indication of potential guidelines that may influence the draft language of the MoCRA GMPs. For example, ISO 22716:2007 - 4.10.3 & 4.10.4 requires specific cleaning and/or sanitizing agents that are verified in effectiveness and are selected and applied according to the specific needs of each facility area. In other words, various areas of your facility may require different cleaning SOPs, and each SOP must be verified in effectiveness. As another example, ISO 22716:2007 - 7.2 outlines the importance of documentation, traceability, quality checks, and batch management, among other requirements. This outlines the push for the industry to move towards “First Time Right” (FTR) ideologies, ensuring operations are audit-ready and products are manufactured and delivered in spec.
Ultimately, these cGMP guidelines help mitigate contamination risk and give companies confidence that they are delivering safe and quality products to their consumers. It must be acknowledged that any new GMP can be intimidating at first. Considering that C&S is a key component of GMPs, this article will walk you through—at a high level—the risks and best practices associated with cleaning procedures for critical processing equipment.
The Cost Of Dirty Equipment
In the digitally connected world we live in, within days, a company’s brand can be negatively impacted via social media when product “defects” make it into the consumer’s hands. Similarly, reports of regulatory audit findings or a recall can damage brand image. These defects or audit findings could have many causes, including residues carried over from the previous production due to inadequate cleaning or flavors and viscosity changes due to microbiological contamination. Ultimately, manufacturers clean to minimise the risk of product contamination. Types of contamination include product cross-contamination, actives cross-contamination, microbial contamination, or even contamination from cleaning products. Given these risks, it is crucial to understand the standards of cleanliness required to ensure product safety and quality. This brings us to the question: “How clean is clean enough?
How Clean Is Clean Enough?
How much to clean to avoid contamination seems subjective and open to interpretation. For discussion, consider that levels of cleaning could be evaluated in three degrees of cleanliness—visually clean, residually clean, or microbially clean. Although these categories of cleanliness are related, each is connected to differing levels of consumer risk. Regulatory bodies charged with ensuring consumer safety have provided some guidance. The following sections will explore each level, the associated risks, and potential solutions.
1. Visually Clean
ISO provides a cleaning standard for the industry that speaks to “visual cleanliness” in ISO 22716 - 2007: Cosmetics—Good Manufacturing Practices (GMP)2, where cleaning is described as “separating and eliminating generally visible dirt from a surface” (section 2.8). This standard directs all visual soil to be removed from an equipment surface.
Of all types of contamination, this standard seems most straightforward to achieve and confirm; however, in practice, this is not always the case. There can be ambiguity in defining the “base state” or starting point. For example, what if the tank is stained, rouged, or somehow damaged, such that no one recalls the beautiful silver luster of the new stainless steel? How would one establish a visually clean standard? Tank remediation or passivation could be performed in a situation like this to return the equipment to a “base state” or a like-new appearance. Alternatively, through testing (residue, microbial) and risk assessment, a quality group and manufacturing group could align on a best-case current state and a new baseline.
So, how does one measure visually clean? Visually clean is a test method requiring a procedure, standardization, and perhaps calibration. Let’s explore three key variables that help define what is considered visually clean: light conditions, surface conditions, and the measurement instrument.
Lighting, or lack of light, can affect the ability to view the surface clearly. Thus, a C&S inspection procedure must include a lighting definition—specifying the light source, bulb type, illumination angle, and so on. Maintenance of that light source could also be necessary, as the condition of the light bulb can deteriorate over time. Sometimes, a preventative maintenance plan for the light source may be necessary.
The condition of the surface inspected is also essential. For instance, a wet surface can appear different from a dry surface and can disguise the presence of soil residue. Thus, the procedure must specify the surface condition, i.e., whether the surface is wet or dry for inspection.
The operator is the primary “instrument” or test device in a visually clean assessment. An operator must be assessed or qualified as capable of performing the test. Visual acuity, corrective lenses, and the ability to distinguish colors can be crucial in qualifying a technician for a visually clean assessment. Once the capability is established, the technician should be trained in the proper inspection techniques following the written procedure.
Once these procedural requirements are defined, success criteria for the test must be established. Since no numeric test result is tied to visual assessment, a visual standard is necessary to ensure consistent measurement against the success criteria. This is often achieved through a visual standard created with photos of acceptable vs. non-acceptable views. Results should be documented following each cleaning. These visual standards support mitigating the risk that accompanies having multiple qualified operators to conduct the visual test. Though paper-based and digital formats are acceptable for housing these procedures, there are many advantages to utilizing a digital format; these advantages include but are not limited to a more interactive and engaging training experience for the operators and robust documentation and historical data traceability for audit readiness.
2. Residually Clean
Even if the equipment is cleaned to a “visually clean” standard, residues present at levels not visible can impact consumer safety. For instance, some actives, such as chemical sunscreen ingredients, are not visually detectable. Also, detergents can leave residues impacting product quality if not rinsed sufficiently, which may not be detectable by visual inspection.
Removing all detectable levels of product, actives, and detergent to ensure a clean surface is not economical for manufacturers, as excessive amounts of time and water may be required. Depending on the industry, regulatory agencies may take a risk-based approach, requiring that residues be reduced to a “safe level” for customer use. Often, that “safe level” is based on a toxicology assessment, ensuring that the residual quantity carried into the next batch is well below an observable effect or an adverse event level. This requires manufacturers to know the residues that could be present, a safe and acceptable residue level, a test method to verify the residue level, and a cleaning process that reliably removes the product, actives, and detergent to that safe level. A cosmetic manufacturer knows their formulation, so they know the actives or formulated ingredients that could remain as residue, and the associated activity or toxicity. They typically have a test method already developed for the active ingredient, as they likely test the final formulation to verify it is within specification. That test method may need to be verified or modified to measure the lower residual actives following cleaning accurately. Frequently, a non-specific test such as the Total Organic Carbon (TOC) test method can verify that non-active formulation ingredients are removed.
The greater unknown for cosmetics manufacturers may lie with the cleaning detergent. A reputable supplier will be able to share the toxicity information for the detergent and appropriate test methods for determining residue. Within the industry, test methods may be specific (identifying the particular chemical component) or non-specific (indicating the presence of a residue but not the particular chemical) based on a toxicity risk assessment. Frequently, detergents used in cosmetics applications are low-risk and can be tested by non-specific methods. Examples include Total Organic Carbon (TOC), conductivity, pH, etc. In any of these cases, lab work is required to provide a correlation or translation from the non-specific method result to the actual residue level. The detergent manufacturer should be able to assist in this development.
While achieving residual cleanliness is crucial for eliminating hidden contaminants, addressing the microbial threats that can compromise product safety is equally important. This brings us to the last level of cleanliness: ensuring equipment is microbially clean.
3. Microbially Clean
Microbial contamination caused by bioburden in the equipment is becoming a more significant concern and interest in the cosmetics industry. Clarifying terms is necessary before we explore options to control microbial contamination. Two terms that confuse the global realm—sanitization and disinfection. In EPA and USP standards, reduction of microbial load is referred to as sanitization, while disinfection is defined as destroying or irreversibly inactivating micro-organisms. For this article, we will use the term sanitization to mean the reduction of microbial load to desired levels, regardless of the geographic location of the application.
Once visual cleanliness and residue levels have been met, there remains potential for microbial residue on equipment that can contaminate a cosmetic product. If microbial contamination is extreme, as in a slimy, discolored area indicating biofilm presence, a visual inspection may detect the issue. Often, microbial contamination is only detected through finished product testing, with high micro counts resulting in batch rejection. Even if history shows no problems with microbial contamination for products manufactured in a facility, current product trends are moving towards more natural ingredients, which may increase the potential for introducing previously unseen microbial flora. In addition, preservative options are shrinking as the EU delists preservatives for use in cosmetic products, meaning less insurance in the formulation to counter microbial growth and contamination.

Without proper cleaning and sanitization procedures, your equipment is at risk of microbial growth, which may present itself as biofilm on the interiors of your equipment
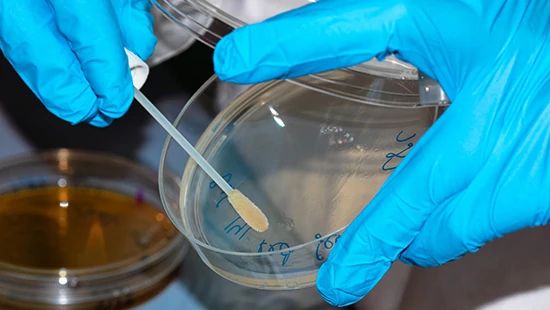
Micro swabbing is one common method to test microbial residues.
Microbial residues can be measured with either micro swabs or rinse water sampling, depending on equipment design and condition. There are unique challenges with microbial residue testing primarily related to access to sample points. Often, the areas in the equipment of highest risk for micro growth are areas that are not easily accessed, such as pipes, equipment joints, or, in some cases, equipment surfaces damaged by gouging or pitting. These areas that are difficult to access for sampling are also the most difficult to effectively sanitize, compounding the risk.
The primary method of controlling microbial residue on cleaned equipment is through a sanitization step. Personal care and cosmetics manufacturers have historically used thermal disinfection to reduce microbial residue. Formulation trends mentioned above are increasing the importance of maintaining good microbial control in the manufacturing equipment. Further pressures to control costs and reduce energy usage result in companies moving from the often-used hot water sanitization to chemical sanitization. Confidence that the thermal process is effective and operating in the validated state highly depends on temperature and time for all equipment contact points. Chemical sanitization is becoming more popular with assured microbiological effectiveness validated using a specific product, concentration, and contact time—often at ambient temperatures and for just a few minutes.
In Summary
Ensuring that personal care and cosmetic products are produced in an environment free from contamination is essential to the health and safety of the consumer. One key step the manufacturer can take is developing a cleaning and sanitizing programme that effectively removes contamination from manufacturing. Those contaminants may be residues from products (actives or not), detergents, or microbial load. Cleaning to a “visually clean” standard is imperative and non-negotiable as a starting point. Development of the standard—the process, acceptance limits, and training—is critical.
We have explored the fact that achieving “visually clean” may not be enough. Residues that are not visible, such as actives residues, detergent residues, or microbioburden, may contribute to contamination impacting product quality. Cleaning and Sanitization programmes must address all these residue types rigorously to provide confidence that product quality is not impacted.
To ensure your products meet the highest cleanliness and safety standards, consider partnering with experts who specialize in contamination control. Doing so can safeguard your brand’s reputation, protect consumer health, and stay ahead of regulatory requirements. Don’t wait for a contamination issue—take proactive steps now to implement a robust cleaning and sanitization programme.

Overcoming Challenges on the Production Floor
Ecolab can optimise your personal care production processes.
Achieve significant savings of time and expense.
References
- Annex 15 of the EU GMP guidelines states “limits for the carryover of product residues should be based on a toxicological evaluation. The justification for the selected limits should be documented in a risk assessment which includes all the supporting references.”
- Source: https://www.epa.gov/pesticide-registration/pesticide-registration-manual-chapter-4-additional-considerations
- Source: USP Chapter 1072



